We are focused on fundamentally understanding and engineering interfacial processes and materials, typically in aqueous systems, as they relate to critical environmental-based health, security, and energy challenges. The following research topics/areas and examples provide an overview of our lab’s major interests and expertise. If you wish to learn more about our research, please contact Professor Fortner at john.fortner@yale.edu.
Advanced Materials for Water Treatment
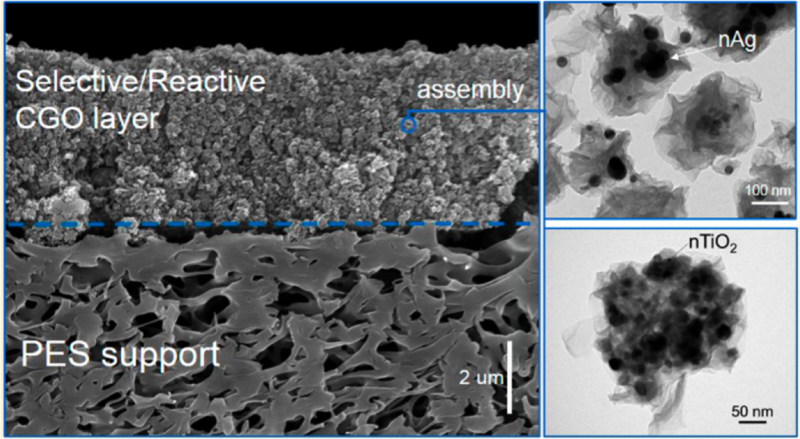
Crumpled graphene oxide (CGO) membrane assembly (top). CGO-nAg (nanoscale silver) nanocomposites. CGO-nTiO2 (nanoscale TiO2) nanocomposites
A major area of research within the Fortner Lab is advancing remediation and water treatment technologies through material-based approaches. As an example, we have recently developed tunable, graphene-based nanocomposites as new, novel photocatalysts, which can be tailored for a range of aqueous-based applications, including selective (photo)redox chemistries.1 Further, with these materials, we have demonstrated a water treatment membranes based on 3-D graphene composites which allow for simultaneous (multifunctional) pollutant degradation, antifouling, and separation processes.2, 3 A U.S. patent has been granted for this technology3 and this work/focus was the basis of awards by the Mindlin Foundation and US NSF through a CAREER award. As another example of research efforts in this area, our lab is also currently part of a DoD SERDP funded team focused on PFAS (per- and polyfluoroalkyl substances) treatment. Specifically, we responsible for the design, development, and evaluation of highly sorptive and reactive (photocatalytic) nanoscale (2- and 3-D) composite materials capable of sequestering and/or degrading PFAS in complicated aqueous matrixes. Other ongoing efforts in this research area within our lab include environmentally responsive- and nano-bio hybrid materials for a range of applications.
Particle-based Environmental Sensing and Separations
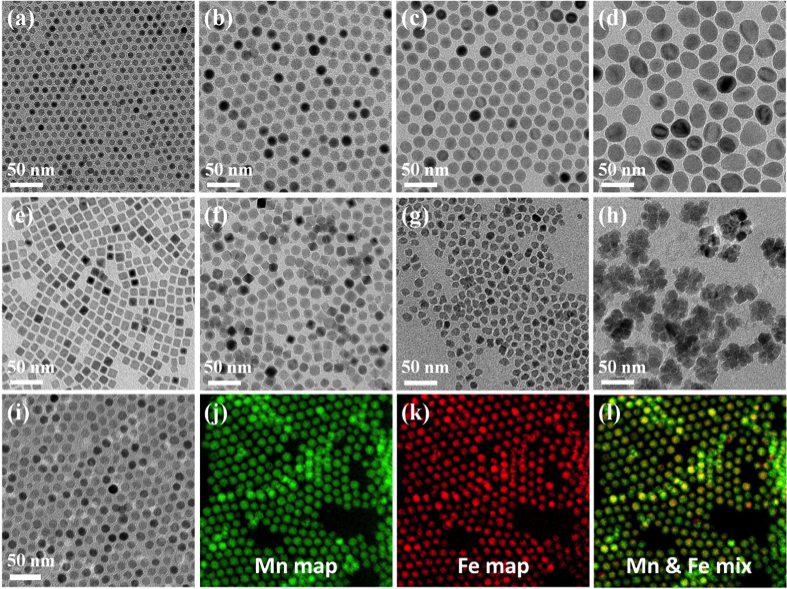
TEM micrographs of superparamagnetic Fe3O4 NPs with different sizes (a-d), TEM micrographs of Fe3O4 NPs with varied shape (e-h), and TEM micrograph of core-shell structured iron oxide-manganese oxide with Gatan Image Filter (GIF) analysis shows mapping position of Mn / Fe (i-l) . All materials shown synthesized in the Fortner Lab.
Over the past decade, our research team has developed and demonstrated significant expertise with regard to novel, magnetic metal oxide nanocrystals (e.g. iron oxide (Fe3O4), manganese oxide (MnxOy), and other ferrite nanocrystals (MnzFe3-zO4)) of varying sizes, shapes, compositions, and precisely tuned surface chemistries for optimized metal/metalloid adsorption, separation, and quantification, among other applications.4-6, 7 As an example, for surface-optimized, superparamagnetic materials, we have reported some of the highest uranium sorption capacities of any material to date.5, 6 Due to high particle monodispersivity and aqueous stabilities, residue geometries can be further arranged as close-packed, sub-micron thin films, which minimize self-shielding and thus allow for truly optimized α-particle detection strategies necessary for (ultra)low-level field-based uranium sensing.8, 9 Our Lab was the lead inventor for a U.S. patent for this suite of technologies.10 Related to this, we are also developing and evaluating similar platform materials for the separation of other inorganic pollutants, including arsenic, chromium, phosphate, and rare earth elements as part of reimagined environmental sensing, separation, and treatment technologies.11, 12-14 These efforts have been broadly supported by the by a number of funding agencies including an NSF project titled, “Platform Nanoscale Sorbents for Advanced Separation and Recovery of Metals and Metalloids in Water” (Fortner PI).
Materials and Processes at the Energy-Water-Food Nexus
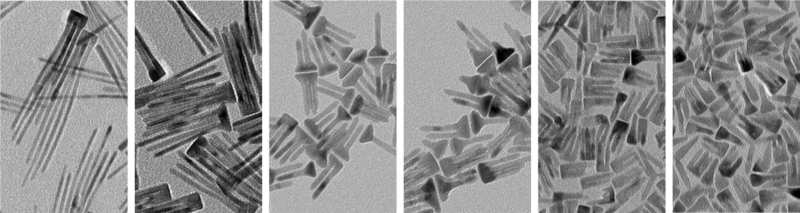
Optimizing Cobalt Oxide Nanorods for H2 evolution (production).31 For these, the mixture of cobalt precursors (mole ratio of cobalt oleate/ cobalt stearate = 7/3) was heated to 320 oC at different ramp rates from 1 to 20 oC/min (1 oC/min (A), 2 oC/min (B), 4 oC/min (C), 8 oC/min (D), 15 oC/min (E) and 20 oC/min (F)) and all nanorods were grown at 320 oC for 30 min. All scale bars are 50 nm.
The Fortner Lab is also focused on critical energy – environmental nexus for which we group has been awarded funding from multiple sources, including the American Chemical Society’s Petroleum Research Fund (PRF, Fortner PI). Research examples at this interface include: The development and characterization of novel, ultra-stable nanoscale T1/T2 contrast agents (ACS PRF).4, 15-17 Quantifying key water chemistry variables (focused on fracking fluids) with regard to down-hole mineral dissolution from two highly productive U.S. shale plays (Eagle Ford and Bakken formations).18, 19 Our team has also developed photocatalytic graphene composite materials for CO2 sorption and transformation via novel reduction pathways.20 Additionally, we have demonstrated surface engineered, magnetic nanomaterials (magnetite core structures with dendritic organic surface ligands, which selectively sorb CO2) for next generation CO2 sorption and separation technologies.21 With regard to the Water – Food nexus, we are developing next generation, particle-based fertilizers through a recently funded USDA project “Impacts of Surface Coating Aging on the Fate, Transport, and Efficacy of Nanofertilizers During Foliar Application” (Fortner PI).
Interfacial Behavior and Chemistry of Advanced Materials in the Environment
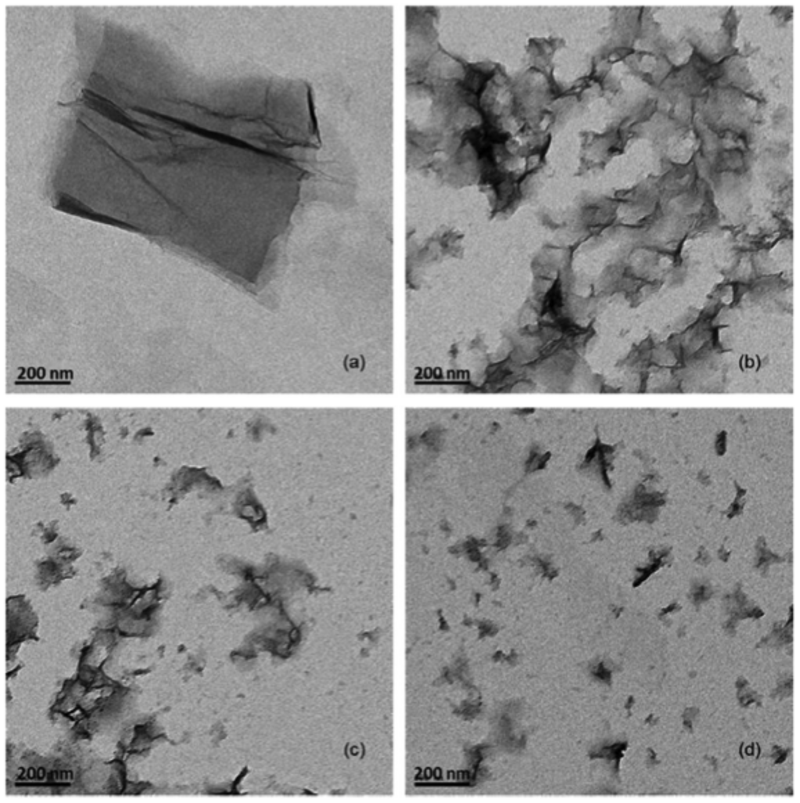
TEM images of graphene oxide transforming in the presence of free chlorine under UVA irradiation (2000 μW/cm2, 2 hr reaction time).32 (a) GO, (b) with 100 mg/L free chlorine at pH 5, (c) with150 mg/L free chlorine at pH 5, (d) with 200 mg/L free chlorine at pH 5.
As industrial-scale manufacturing is now a reality for a range of advanced materials, fundamental understanding of (interfacial) environmental chemistries and potential implications is necessary for sustainable application. Within this framework, which continues to be refined, the Fortner Lab has made significant contributions towards such understanding for a range of engineered carbon-based materials, including fullerenes, carbon nanotubes (CNT), and graphene(s), as well as environmental aging processes for surface stabilized, nanomaterials. A number of key reports in this area have come from his lab including the first observation and characterization of fullerene (as an oxidized derivative) reduction in water22; the first report of (photo)oxidation transformation of fullerane (a hydrogenated fullerene)23; and the first comprehensive reports of the chlorination of aggregated fullerenes under dark (ground state) conditions and under UVA light in the presence of common environmental oxidants.24-26 Other related research includes evaluating the atmospheric chemistry of engineered nanomaterials (focused on fullerenes, NSF funded, Fortner PI).27
More recently, our team has focused on the aqueous chemistry of graphene (and related graphene oxides) along with surface stabilized inorganic nanomaterials. We have documented a range of material transformations, including product characterization, and kinetics under light and/or with relevant oxidative reactants, including free chlorine32, and environmental reductants. We demonstrated that particle shape and surface chemistry of graphene composites are key to understanding environmental behavior/stability.28, 29 In collaboration with Brown University through a NSF grant titled, “Impacts of Surface Coating Aging on Nanomaterial Fate and Transport in Porous Media,” we identified and correlated material (iron oxide Fe3O4 and silver nanoparticles) stability as a function of surface aging processes under relevant environmental oxidative conditions (including UVA irradiation).17, 30 Further, in collaboration with Brown and Auburn Universities we are assessing the role of biosurfactants with regard to the fate and transport of nanomaterials “Effects of Nano-Bio Interactions on Nanoparticle Fate and Transport in Porous Media.” Other ongoing efforts in these related areas include fundamentally understanding the dynamics of soft nanoscale materials in the environment and other biological-based interactions.
Work Cited
1. Jiang, Y.; Wang, W. N.; Biswas, P.; Fortner, J. D., Facile Aerosol Synthesis and Characterization of Ternary Crumpled Graphene-TiO2-Magnetite Nanocomposites for Advanced Water Treatment. Acs Appl Mater Inter 2014, 6, (14), 11766-11774.
2. Jiang, Y.; Liu, D.; Cho, M.; Lee, S. S.; Zhang, F.; Biswas, P.; Fortner, J. D., In Situ Photocatalytic Synthesis of Ag Nanoparticles on Crumpled Graphene Oxide Composite Membranes for Filtration and Disinfection Applications. Environmental Science and Technology 2015, In Review.
3. Jiang, Y.; Wang, W.-N.; Biswas, P.; Fortner, J. D. Composite Nanostructures Having a Crumpled Graphene Oxide Shell 2015.
4. Li, W. L.; Mayo, J. T.; Benoit, D. N.; Troyer, L. D.; Lewicka, Z. A.; Lafferty, B. J.; Catalano, J. G.; Lee, S. S.; Colvin, V. L.; Fortner, J. D., Engineered superparamagnetic iron oxide nanoparticles for ultra-enhanced uranium separation and sensing. J Mater Chem A 2016, 4, (39), 15022-15029.
5. Lee, S. S.; Li, W. L.; Kim, C.; Cho, M. J.; Lafferty, B. J.; Fortner, J. D., Surface functionalized manganese ferrite nanocrystals for enhanced uranium sorption and separation in water. J Mater Chem A 2015, 3, (43), 21930-21939.
6. Lee, S. S.; Li, W. L.; Kim, C.; Cho, M.; Catalano, J. G.; Lafferty, B. J.; Decuzzi, P.; Fortner, J. D., Engineered manganese oxide nanocrystals for enhanced uranyl sorption and separation. Environ Sci-Nano 2015, 2, (5), 500-508.
7. Li, W. L.; Troyer, L. D.; Lee, S. S.; Wu, J. W.; Kim, C.; Lafferty, B. J.; Catalano, J. G.; Fortner, J. D., Engineering Nanoscale Iron Oxides for Uranyl Sorption and Separation: Optimization of Particle Core Size and Bilayer Surface Coatings. Acs Appl Mater Inter 2017, 9, (15), 13163-13172.
8. Li, W.; Mayo, J. T.; Benoit, D. N.; Troyer, L. D.; Lewicka, Z. A.; Lafferty, B. J.; Catalano, J. G.; Lee, S. S.; Colvin, V. L.; Fortner, J. D., Engineered Superparamagnetic Nanoparticles for Enhanced Uranium Separation and Sensing. Journal of Materials Chemistry A, 2016, 4, (39), 15022-15029.
9. Kim, C.; Lee, S. S.; Reinhart, B. J.; Cho, M. J.; Lafferty, B. J.; Li, W. L.; Fortner, J. D., Surface-optimized core-shell nanocomposites (Fe3O4@MnxFeyO4) for ultra-high uranium sorption and low-field separation in water. Environ Sci-Nano 2018, 5, (10), 2252-2256.
10. Lee, S. S.; Lafferty, B. J.; Li, W.; Fortner, J. D. Engineered Nanoparticles for Aqueous Applications. 2015.
11. Farrell, J. W.; Fortner, J.; Work, S.; Avendano, C.; Gonzalez-Pech, N. I.; Araiza, R. Z.; Li, Q. L.; Alvarez, P. J. J.; Colvin, V.; Kan, A.; Tomson, M., Arsenic Removal by Nanoscale Magnetite in Guanajuato, Mexico. Environ Eng Sci 2014, 31, (7), 393-402.
12. Kim, C.; Lee, S. S.; Lafferty, B. J.; Giammar, D. E.; Fortner, J. D., Engineered superparamagnetic nanomaterials for arsenic(V) and chromium(VI) sorption and separation: quantifying the rote of organic surface coatings. Environ Sci-Nano 2018, 5, (2), 556-563.
13. Wu, B. L.; Fang, L. P.; Fortner, J. D.; Guan, X. H.; Lo, I. M. C., Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)(3)/Fe3O4 nanocomposites. Water Res 2017, 126, 179-188.
14. Pan, Z. Z.; Li, W. L.; Fortner, J. D.; Giammar, D. E., Measurement and Surface Complexation Modeling of U(VI) Adsorption to Engineered Iron Oxide Nanoparticles. Environ Sci Technol 2017, 51, (16), 9219-9226.
15. Li, W. L.; Hinton, C. H.; Lee, S. S.; Wu, J. W.; Fortner, J. D., Surface engineering superparamagnetic nanoparticles for aqueous applications: design and characterization of tailored organic bilayers. Environ Sci-Nano 2016, 3, (1), 85-93.
16. Li, W. L.; Liu, D.; Wu, J. W.; Kim, C.; Fortner, J. D., Aqueous Aggregation and Surface Deposition Processes of Engineered Superparamagnetic Iron Oxide Nanoparticles for Environmental Applications. Environ Sci Technol 2014, 48, (20), 11892-11900.
17. Li, W.; Lee, S. S.; Mittelman, A. M.; Liu, D.; Wu, J.; Hinton, C. H.; Abriola, L. M.; Pennell, K. D.; Fortner, J. D., Aqueous Aggregation Behavior of Engineered Superparamagnetic Iron Oxide Nanoparticles: Effects of Oxidative Surface Aging. Environmental Science and Technology 2016, ASAP article.
18. Wang, L.; Burns, S.; Giammar, D. E.; Fortner, J. D., Element mobilization from Bakken shales as a function of water chemistry. Chemosphere 2016, 149, 286-293.
19. Wang, L.; Fortner, J. D.; Giammar, D. E., Impact of Water Chemistry on Element Mobilization from Eagle Ford Shale. Environ Eng Sci 2015, 32, (4), 310-320.
20. Wang, W. N.; Jiang, Y.; Fortner, J. D.; Biswas, P., Nanostructured Graphene-Titanium Dioxide Composites Synthesized by a Single-Step Aerosol Process for Photoreduction of Carbon Dioxide. Environ Eng Sci 2014, 31, (7), 428-434.
21. Li, W.; Wu, J.; Lee, S. S.; Fortner, J. D., Surface Tunable Magnetic Nano-sorbents for Carbon Dioxide Sorption and Separation. Chemical Engineering Journal 2016, accepted and in press
22. Wu, J. W.; Alemany, L. B.; Li, W. L.; Petrie, L.; Welker, C.; Fortner, J. D., Reduction of Hydroxylated Fullerene (Fullerol) in Water by Zinc: Reaction and Hemiketal Product Characterization. Environ Sci Technol 2014, 48, (13), 7384-7392.
23. Wu, J. W.; Goodwin, D. G.; Peter, K.; Benoit, D.; Li, W. L.; Fairbrother, D. H.; Fortner, J. D., Photo-Oxidation of Hydrogenated Fullerene (Fullerane) in Water. Environ Sci Tech Let 2014, 1, (12), 490-494.
24. Wu, J. W.; Benoit, D.; Lee, S. S.; Li, W. L.; Fortner, J. D., Ground State Reactions of nC(60) with Free Chlorine in Water. Environ Sci Technol 2016, 50, (2), 721-731.
25. Wu, J.; Alemany, L. B.; Li, W.; Benoit, D.; Fortner, J. D., Photoenhanced Transformation of Hydroxylated Fullerene (Fullerol) by Free Chlorine. Environmental Science: Nano 2016, in revision
26. Ou, Y. N.; Wu, J. W.; Meyer, J. R.; Foston, M.; Fortner, J. D.; Li, W. L., Photoenhanced oxidation of nC(60) in water: Exploring H2O2 and hydroxyl radical based reactions. Chemical Engineering Journal 2019, 360, 665-672.
27. Mitroo, D.; Wu, J. W.; Colletti, P. F.; Lee, S. S.; Walker, M. J.; Brune, W. H.; Williams, B. J.; Fortner, J. D., Atmospheric Reactivity of Fullerene (C-60) Aerosols. Acs Earth and Space Chemistry 2018, 2, (2), 95-102.
28. Jiang, Y.; Raliya, R.; Fortner, J. D.; Biswas, P., Graphene Oxides in Water: Correlating Morphology and Surface Chemistry with Aggregation Behavior. Environ Sci Technol 2016, 50, (13), 6964-6973.
29. Jiang, Y.; Raliya, R.; Liao, P.; Biswas, P.; Fortner, J. D., Graphene oxides in water: assessing stability as a function of material and natural organic matter properties. Environ Sci-Nano 2017, 4, (7), 1484-1493.
30. Mittelman, A. M.; Fortner, J. D.; Pennell, K. D., Effects of ultraviolet light on silver nanoparticle mobility and dissolution. Environ Sci-Nano 2015, 2, (6), 683-691.
31. Changwoo Kim, Seung Soo Lee, Wenlu Li, and John D. Fortner, “Towards Optimizing Cobalt Based Metal Oxide Nanocrystals for Hydrogen Generation via NaBH4 Hydrolysis,” Applied Catalysis A: General, 589, p.117303, 2020.
32. Siyuan An, Jiewei Wu, Yao Nie, Wenlu Li, John D. Fortner, “Free chlorine induced phototransformation of graphene oxide in water: Reaction kinetics and product characterization,” Chemical Engineering Journal, 381, p.122609, 2020.